FOR IMMEDIATE RELEASE
Health Startup Unveils $99 At-Home Fertility Kit, Slashes Diagnostic Cost by 80%
FDA-cleared device couples smartphone AI with lab-grade hormone analytics to deliver same-day ovulation and ovarian-reserve insights without clinic visits.
SAN FRANCISCO – November 21, 2025
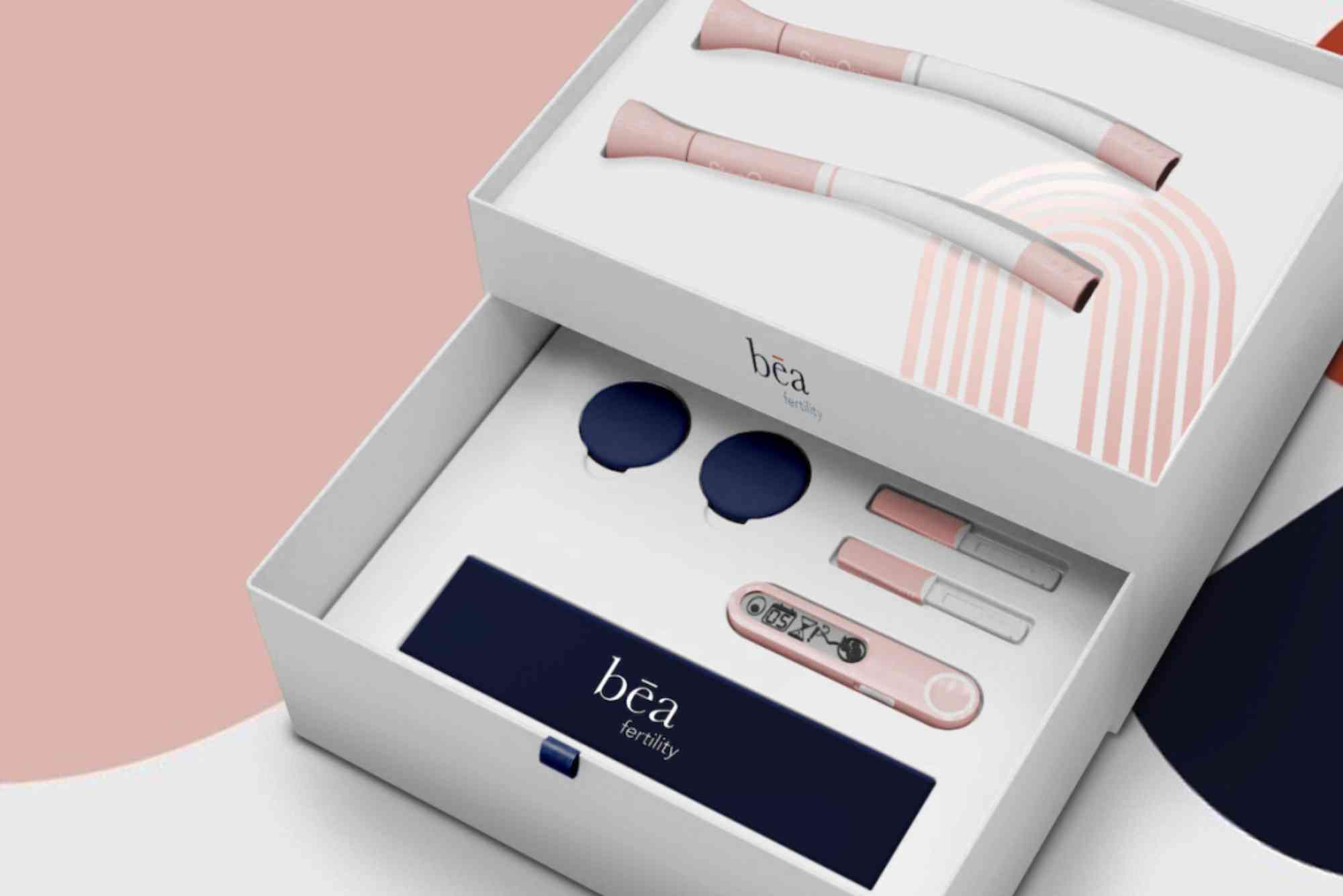
Bay-area fem-tech company Nurture NOW Inc. today launched the NurtureNOW Fertility Monitor, a pocket-sized, single-use cartridge that quantifies luteinizing hormone (LH), estrogen and anti-Müllerian hormone (AMH) from two drops of finger-stick blood. Results are processed by a phone-based AI engine and returned within 15 minutes at a retail price of $99—up to 80 percent less than comparable laboratory panels.
The debut comes as demand for at-home reproductive testing surges. The global ovulation-testing market is projected to grow from $2.8 billion in 2025 to $4.3 billion by 2032, a compound annual growth rate of 6.2 percent, driven by delayed child-bearing and a shift toward self-managed care . Nearly one in three U.S. women of reproductive age report difficulty accessing in-clinic fertility services, according to 2025 survey data from virtual-care network Maven Clinic .
“We designed NurtureNOW to close the gap between realizing you want a child and actually understanding your body,” said Dr. Leila Farzin, co-founder and chief executive. “By miniaturizing lab-grade chemiluminescence sensors and pairing them with an AI model trained on more than 250,000 hormonal profiles, we can deliver clinically accurate, personalized fertility windows without the three-week wait for a doctor’s appointment.”
Each kit contains a sterile lancet, a disposable microfluidic cartridge and a QR-coded sleeve that launches the NurtureNOW app. After imaging the cartridge, the app graphs three-hormone trends, predicts ovulation up to 48 hours in advance and flags diminished ovarian reserve if AMH falls below 1.0 ng/mL, the threshold recommended by the American Society for Reproductive Medicine. Data can be exported as a PDF for physician consultation or uploaded directly to electronic health records through the Apple Health integration.
A pivotal trial completed at Stanford University’s Fertility & Reproductive Health Center this summer compared the kit with standard serum assays in 412 women aged 21–43. The study found 96.4 percent positive percent agreement for LH surge detection and 94.7 percent correlation for AMH quantification, meeting FDA 510(k) criteria for waived, over-the-counter use. The clearance, granted in September, positions NurtureNOW as the first multi-hormone, blood-based fertility monitor cleared for OTC sale in the United States.
Investors have taken notice. The company closed a $22 million Series A round in October led by Andreessen Horowitz’s Bio + Health fund, with participation from GV and Pivotal Ventures. Funds will scale manufacturing at NurtureNOW’s Sunnyvale facility and underwrite a national tele-health partner network that offers same-day follow-up with board-certified reproductive endocrinologists.
“Cost is the silent barrier to family-building,” said Dr. Farzin. “When a single AMH test averages $250 out-of-pocket and insurance coverage remains spotty, many people simply never get assessed. At $99, our kit is cheaper than most ovulation predictor strip packs used over six cycles, yet it answers both ‘When do I ovulate?’ and ‘How much time do I have?’ in one test.”
The product will debut online at nurturenow.com and in 1,200 CVS Pharmacy locations across California, Texas and New York beginning December 1, with nationwide rollout planned for Q1 2026. A subscription option at $79 per quarterly kit includes unlimited in-app tele-consults and automatic refills synced to cycle length.
Media Contact
Sarha Al-Mansoori
Director of Corporate Communications
G42
Email: media@g42.ai
Phone: +971 2555 0100
Website: www.g42.ai