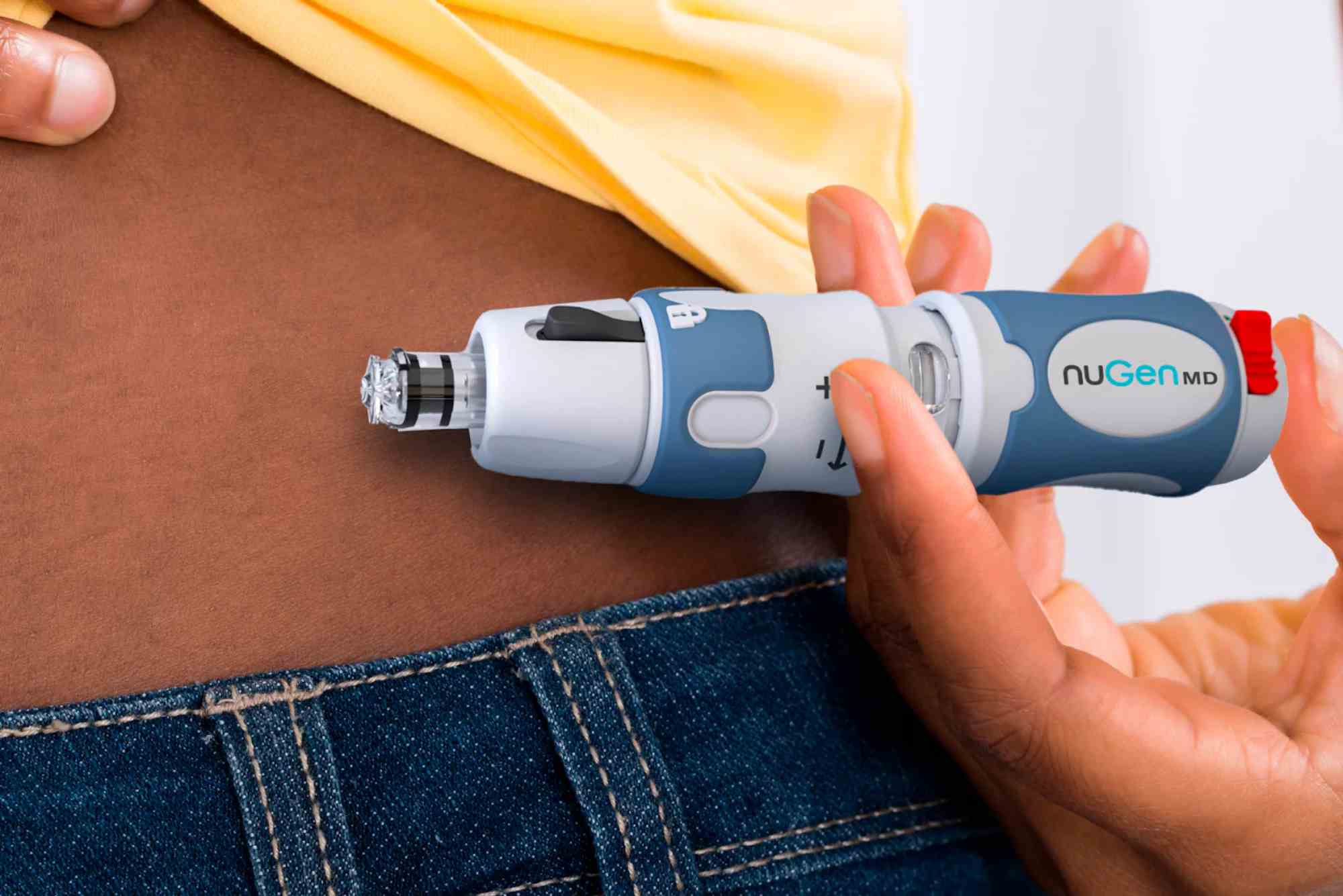
Beta Bionics Unveils Mint Patch Pump Prototype, Advancing Needle-Free Insulin Delivery
SAN DIEGO, June 24, 2025 – Beta Bionics, Inc., a commercial-stage medical technology company specializing in automated insulin delivery systems, today unveiled Mint, a needle-free patch pump prototype designed to simplify diabetes management through tubeless, phone-free operation. The device represents the company’s first foray into fully disposable insulin delivery hardware, complementing its existing portfolio of bionic pancreas solutions.
The Mint prototype addresses a critical unmet need in diabetes care: reducing the physical and psychological burden of multiple daily injections and cumbersome insulin pump tubing. According to the Centers for Disease Control and Prevention, 37.3 million Americans live with diabetes, many requiring intensive insulin therapy that demands 4-6 injections daily or traditional pump management with infusion sets that must be changed every 2-3 days. Industry analysts note that patch pumps are gaining traction as they eliminate tubing entirely, offering greater discretion and lifestyle flexibility. As reported by MedTech Dive, Beta Bionics previewed the Mint system at the American Diabetes Association’s 2025 Scientific Sessions, positioning it as a thoughtful evolution of existing patch pump architectures .
The Mint device combines disposable and reusable components, with a durable element designed to last approximately two years requiring only periodic battery replacement. Each three-day disposable pod accommodates up to 200 units of insulin—the standard capacity for patch pumps—and features what company engineers describe as the smallest cannula yet developed for this category. Unlike smartphone-dependent systems that require constant Bluetooth connectivity, Mint operates as a standalone insulin delivery device, reducing potential points of failure and simplifying the user experience for populations with limited technology access or proficiency.
“Mint embodies our commitment to patient-centric design by eliminating the most common pain points in insulin therapy,” said Dr. Edward Damiano, CEO and co-founder of Beta Bionics. “We’ve created a system that doesn’t ask patients to choose between convenience and clinical efficacy. The three-day wear time, paired with tubeless freedom and autonomous operation, gives people with diabetes back the spontaneity that this condition too often restricts.”
Market data underscores the commercial opportunity for innovative insulin delivery systems. The global continuous glucose monitoring market is expanding at a compound annual growth rate of 8.62 percent, projected to reach $13.6 billion by 2034, according to GlobalData analysis . Meanwhile, the broader wearable medical device market is forecast to grow from $99.5 billion in 2022 to $290.6 billion by 2030, reflecting accelerating demand for minimally invasive health technologies . Recent clinical research demonstrates that transdermal insulin delivery methods, including patch systems and microneedle arrays, achieve superior or comparable glycemic control to subcutaneous injections in 61 percent of comparative studies, while showing equal or better tolerability across all evaluated trials .
Beta Bionics has not announced a commercialization timeline for Mint, though industry observers expect a phased development approach similar to the company’s iLet Bionic Pancreas, which received FDA clearance in 2023. The company will need to conduct clinical investigations to validate the prototype’s safety and efficacy profile before submitting for regulatory review. The device’s semi-reusable architecture may offer manufacturing efficiencies compared to fully disposable competitors, potentially improving accessibility in cost-sensitive markets.
About Beta Bionics
Beta Bionics, Inc. is a commercial-stage medical technology company dedicated to improving outcomes for people living with diabetes. The company’s flagship iLet Bionic Pancreas is the only FDA-cleared insulin-only configuration that automatically delivers and adjusts insulin doses based on CGM data without requiring carbohydrate counting. Beta Bionics went public in January 2025 and is traded on the NASDAQ under the ticker symbol BBNX. The company is headquartered in San Diego, California, with additional operations in Boston, Massachusetts.
Media Contact:
Sarha Al-Mansoori
Director of Corporate Communications
G42
Email: media@g42.ai
Phone: +971 2555 0100
Website:www.g42.ai